
A Clinical Trial Management System (CTMS) is a software application that facilitates the planning, management, and tracking of clinical trials. It is designed to streamline and automate the operational and administrative aspects of clinical trials, including study start-up, patient enrollment, data management, monitoring, and reporting.
One of the main challenges faced by Clinical trial management systems (CTMS) is the complexity of the clinical trial process. Clinical trials involve many stakeholders, including study sponsors, investigators, study coordinators, and regulatory bodies.
The system was designed to automate key processes such as study start-up, patient enrollment, data management, and monitoring. The CTMS was also integrated with other systems, such as electronic data capture (EDC) systems, to streamline the flow of data.
Helps set up and manage studies, including study protocols, timelines, budgets, and resources
Helps manage site information, including site profiles, feasibility assessments, site contracts, and payments
Helps manage participant enrollment, randomization, and tracking, as well as patient visit scheduling and tracking
Helps manage investigator information, including profiles, CVs, and training records, as well as communication and collaboration tools
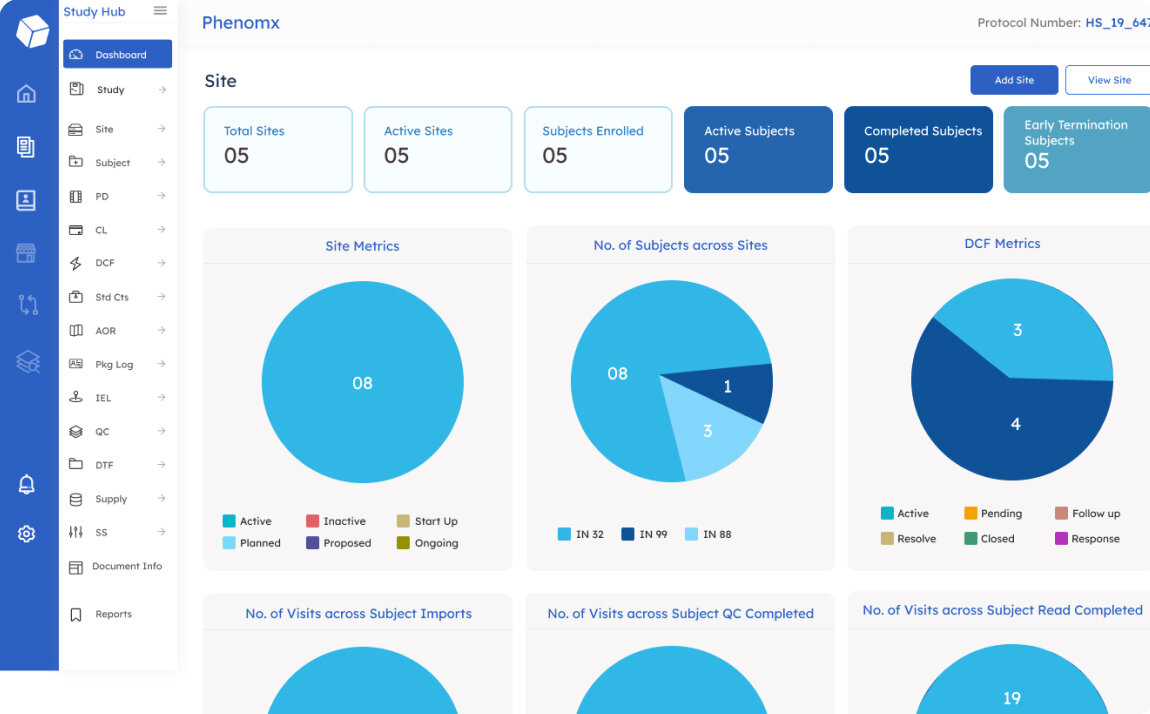
Helps track study progress and compliance, generate reports and dashboards, and manage communication between study team members
Helps store and manage study documents, including protocols, informed consent forms, regulatory documents, and study reports
Generate a more engaged audience
Page interface keeps audience engaged
and improves customer loyalty.
Improve conversion rates
Potential customers have greater chances
of conversion.
Boost brand loyalty
Create a better impression
about your brand.
Build a strong brand identity
Develop a unique identity for your business with
an incredible UI/UX.